Introduction:
Being a part of an India family, there are lots of fried dishes that are made on festivals. On the occasion of Diwali, other family members had come to our home. We had planned to make Jalebi on our own. I was in the kitchen and trying to learn the process of making Jalebi. My mother told me that frying happens in different batches. I was tempted to use the oil left over after each frying as the medium in which the next batch of Jalebi dough would be into the oil. When denied by my parents to not do so owing to the health risks of such a procedure, I decided to think deeper into the changes that take place in an oil medium during frying, which in turn, affects the frying quality as well as the health hazards of the food being cooked. Being an IB chemistry HL student, I decided to do an investigation on what actually happens inside the molecule when you heat/fry the oil for longer periods.
In order to carry out my exploration, my first aim was to understand qualitatively how successive frying affects an oil. On conducting research, I found that frying causes thermal oxidation of the oil, increasing its saturation. This change is largely responsible for the effects on health standards and food quality. Since the saturation of oil can be measured by its iodine value (the mass of iodine (in grams) required to saturate 100 g of the oil), I decided to measure how the iodine value of oils changes after each batch of frying/heating.
Additionally, in order to determine which oils are most susceptible to change on frying, I decided to carry out my experiment for five different commonly-used oils: palm kernel oil, olive, castor, groundnut and palm. Corresponding to the event which prompted this exploration, Jalebis were maintained to be the food used in each frying procedure.
Research Question:
How does temperature in terms of heating/frying processes of Jalebis affect the iodine values of vegetable oils after a measured amount of time.
Background Information:
Overview of the Chemical changes that occur in oil:
During the frying period, as the heat is transferred from the oil to the food, the water evaporates and oil is absorbed by the food which causes hydrolysis. Moreover, since the oil remains in contact with air, the high temperature in the fryer causes several oxidation reactions to take place in the oil. The compounds formed in the oil by these reactions might undergo polymerisation or further hydrolysis and oxidation, depending on the creation of suitable conditions.
Effect of hydrolysis reactions:
When the food is being fried in hot oil, the moisture from the food forms steam and evaporates with a bubbling action and gradually subsides as the food is fried. Water, steam and oxygen start the chemical reactions in the frying oil and food. Water being a weak nucleophile tends to attack the ester linkage of triacylglycerols and produces di- and monoacylglycerols, glycerol, and free fatty acids.
Since nucleophiles attack high electron density areas more readily, unsaturated compounds, which have a π-electron cloud due to their double or triple bonds, are more likely to undergo hydrolysis reactions. Thus, oils having a greater concentration of unsaturated fatty acids exhibit a greater production of hydrolysis compounds during frying.
Effect of Oxidation reactions:
Oxidation reactions involve the conversion of the unsaturated compounds in oil to saturated compounds. Therefore, a greater concentration of unsaturated fatty acids in oil increases its susceptibility to oxidation. Ordinary oxygen in air is a diradical compound. Radical oxygen requires radical oil for the oxidation of oil. Oil should be in a radical state to react with radical oxygen for oil oxidation reaction. The hydrogen with the weakest bond on the carbon of oil will be removed first to become radical. Therefore, since oxidation reactions during frying decrease the unsaturation of an oil, the iodine number of oils is expected to decrease with successive frying. Moreover, since higher unsaturation favours greater oxidation, oils with high initial iodine values are expected to show greater decreases after frying than those with lower iodine value.
Effect of Composition and Food quality and health:
Effect of food quality:
Effect of hydrolysis reaction:
Since the products formed during the hydrolysis of triacylglycerol (di- and monoacylglycerols, glycerol and free fatty acids) produce off-flavor in the cooked food, oils undergoing hydrolysis reactions more readily are not preferable from the perspective of food quality. As mentioned above the Effect of Hydrolysis Reactions, oils with a higher degree of unsaturation are more susceptible to hydrolysis, and are therefore, less preferable when taste is concerned.
Effect of oxidation reactions:
As established above the Effect of Oxidation Reactions, the degree of saturation of an oil increase with successive frying due to the oxidation reactions taking place inside it. Since susceptibility to hydrolysis decreases as saturation increases, taste desirability is expected to improve through successive frying.
Effect on Health:
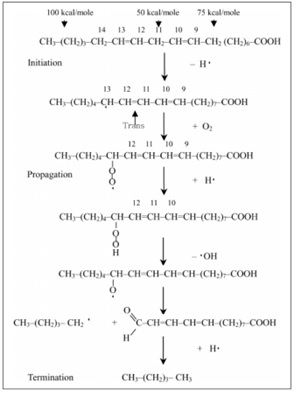
The oxidation reactions taking place inside the oil lead to the production of trans fats which increase bad cholesterol level (LDL) and lower good cholesterol levels (HDL), thus, increasing risk of heart disease, stroke and developing Type 2 Diabetes. The initiation of a possible free radical mechanism that produces such trans fats during the frying process is shown in Figure 1 below.
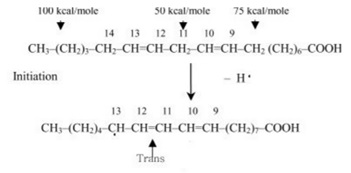
Fig 1: Trans fats are produced during the oxidation of oil during frying (as first seen in initiation reaction), making frying in batches harmful for health.
Hypothesis:
While iodine value will decrease through batches of frying, the change in iodine value will decrease with the number of fries. Moreover, oils with greater initial iodine values are expected to show greater changes in iodine values than oils with lower initial iodine value. So, at the end of the process, the one with the lowest Iodine value would be the worst for our health the one with highest would be best amongst the 5 oils that I have chosen.
Variables:
Independent:
- Mass of oil
- Initial iodine value of oil
Dependent:
- Final iodine value of oil after just reaching the boiling point.
Methodology:
First, I decided the Independent, Dependent, and Control variables.
| Independent variables | Dependent variables | Control variables |
| Type of Vegetable oil | Iodine value after boiling point | Ratio of food to oil |
| Jalebi dough | Boiling Temperature of oil. | |
| Mass of oil |
Method to find the Iodine value of an oil:
Apparatus required:
- Burette (25 ml)
- Retort stand and clamp
- 100 ml measuring cylinders
- 100 ml beakers
- 10ml measuring cylinder
- 100ml volumetric flask
- Stoppered bottle (250 ml)
- Analytical Balance
Materials required:
- 5g of palm oil
- 5g of groundnut oil
- 5g of olive oil
- 5g of castor oil
- 5g of palm kernel oil
- Wij’s reagent (ICl 0.2M)
- Sodium thiosulphate (Na2S2O3 0.1M)
- Potassium Iodide (KI 10g/l)
- Starch indictor (1g/l)
- Chloroform (10 ml)
Iodine value were determined according to the titrimetric method of Pearson (1970). 5g of the vegetable oil sample were weighed into 250 mL conical flask and 10 mL of chloroform were added to dissolve the oil sample, 25 mL of the Wijs reagent (ICl) were added. The flasks were sealed, shaken thoroughly and placed in a fume cupboard for 11 hours. After the 11 hours, 10 mL of 10% potassium iodide (KI) were added to the sample solution. The sample solution was immediately titrated with 0.2M sodium thiosulphate (Na2S2O3). The samples were titrated to a yellow straw colour and then 1 mL of the 1% starch solution were added to the solution which results to a dark-blue colouration of the solution and titration continues until the dark-blue colour disappears leaving behind a clear solution with thorough shaking of the conical flask throughout the titration process in order to ensure that all the iodine were removed from the chloroform layer.
At the same time, a blank solution was set up containing only 25 ml of Wij’s reagent and 10ml of chloroform titrated with 0.2M sodium thiosulphate until a clear solution were observed. The titration processes were repeated with the other four oil samples.
The volume of the sodium thiosulphate in the burette were recorded in a data table. One blank of each trial and one test sample of each trial were utilized. The difference between the blank (B) and test (T) reading (B-T), gives the number of ml of 0.2M sodium thiosulphate needed to react with the equivalent volume of iodine.
The iodine value from the above experiment were calculated from the average titer volume of sodium thiosulphate (Na2S2O3) used in the titration of both the sample and the blank and were plugged into the relation.
Iodine Number = M (B- T) (12.69)
W
Where “M” = Molarity of the standard sodium thiosulphate solution,
Where “T” = the volume of sodium thiosulphate required to titrate the test solution containing the oil samples.
W = the weight in grams of the sample
Where “126.9” is the molecular weight of iodine per 100g of the oil sample
Final results and Conclusion:
The results of this research work are summarized using tables as shown below:
Table I: Titration values of Groundnut oil
| Titration | Blank | 1 | 2 | 3 |
| Final Burette Reading (ml) | 170.00 | 0.60 | 0.50 | 0.60 |
| Initial Burette Reading (ml) | 0.00 | 0.00 | 0.00 | 0.00 |
| Titre (ml) | 170.00 | 0.60 | 0.50 | 0.60 |
Average titre = 0.57 ml
Table II: Titration values of Olive oil
| Titration | Blank | 1 | 2 | 3 |
| Final Burette Reading (ml) | 170.00 | 10.40 | 10.30 | 10.50 |
| Initial Burette Reading (ml) | 0.00 | 0.00 | 0.00 | 0.00 |
| Titre (ml) | 170.00 | 10.40 | 10.30 | 10.50 |
Average titre = 10.40 ml
Table III: Titration values of Palm oil
| Titration | Blank | 1 | 2 | 3 |
| Final Burette Reading (ml) | 170.00 | 63.60 | 63.80 | 64.00 |
| Initial Burette Reading (ml) | 0.00 | 0.00 | 0.00 | 0.00 |
| Titre (ml) | 170.00 | 63.60 | 63.80 | 64.00 |
Average titre = 63.80 ml
Table IV: Titration values of Palm Kernel oil
| Titration | Blank | 1 | 2 | 3 |
| Final Burette Reading (ml) | 170.00 | 97.10 | 98.00 | 97.80 |
| Initial Burette Reading (ml) | 0.00 | 0.00 | 0.00 | 0.00 |
| Titre (ml) | 170.00 | 97.10 | 98.00 | 97.80 |
Average titre = 97.63 ml
Table V: Titration values of Coconut oil
| Titration | Blank | 1 | 2 | 3 |
| Final Burette Reading (ml) | 170.00 | 150.30 | 150.10 | 150.50 |
| Initial Burette Reading (ml) | 0.00 | 0.00 | 0.00 | 0.00 |
| Titre (ml) | 170.00 | 150.30 | 150.10 | 150.50 |
Average titre = 150.30 ml
Table VI: Showing the Iodine Values of Five Different Vegetable Oils
| Vegetable oils | Iodine value (g I2/100g) | Food Safety and Standards Authority of India (FSSAI 2011) |
| Groundnut oil | 86.00 | 85 – 99 |
| Olive oil | 81.01 | 75 – 94 |
| Palm oil | 53.91 | 45 – 56 |
| Palm kernel oil | 36.74 | 10 – 23 |
| Coconut oil | 10.00 | 7.5 – 10 |
The above pie chart shows the Iodine values of the 5 vegetable oils.
Conclusion:
Based on findings and result so far, it is evidently clear that iodine value of oil determines how the oil is defined, its uses and applications. Iodine value is an indicator of the presence of double bonds in the molecular structure of fats and oils, which influences the long-term stability properties of the oil (i.e., important for storage). It has been reported that oil with low iodine value improves the stability and good yield of the liquid oil.
The more iodine is attached, the higher is the iodine value, and the more reactive, less stable, softer, and more susceptible to oxidation and rancidification is the oil.
The iodine values of the five vegetable oil samples analyzed were less than 115, therefore they are considered to be non-drying, and at such they can be used for soap making (hard soaps) and in food products. Non-drying oil is oil which does not harden when it is exposed to air. This is as opposed to a drying oil, which hardens (through polymerization) completely, or semi-drying oil, which partially hardens. Oils with an iodine number of less than 115 are considered non-drying. The result of these findings shows that the oils are of good nutritional value and are good for industrial applications; hence the oils pose no significant health risks to consumers.